Fungicide Resistance
Fungicide resistance is a relative term that describes the reduction in sensitivity to a fungicide in a fungal population beyond natural variation. The natural variation of a fungal pathogen population is described as the baseline sensitivity. Baseline sensitivities are derived from a sample of pathogen individuals that were never exposed to the fungicide. Generally, a normal distribution of variation occurs that may be skewed based on the pathogen and type of chemistry or selection pressure. Resistance is an inheritable genetic trait that is distinguished from adaptation where the same individual reverts back to sensitivity to the fungicide after some period of absence of exposure. Field-resistance (practical resistance) is the reduction in sensitivity in the pathogen that is accompanied by crop losses.
Resistance frequency is the relative incidence of a less sensitive variant within a population of individuals that has the ability to survive under the selection pressure of a fungicide. Variants arise from genetic mutations that are continuously and spontaneously occurring within populations of organisms. Some mutations are detrimental, whereas others may allow survival of individuals under a specific stress such as the presence of a toxicant (i.e., fungicide). Resistance frequencies are generally very low numbers (e.g., 1 in millions) and as such, resistance is a rare event. Still, fungi are able to reproduce in great numbers. Thus, although fungicides may eliminate most of the population, a few survivors can replace the sensitive population in a relatively short time. Once resistance is selected, then the resistance factor or the magnitude of resistance can be calculated as compared to the baseline sensitivity level.
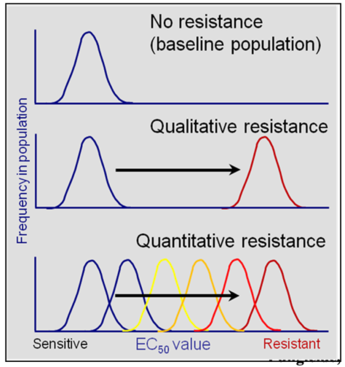
Fungicide resistance can be further characterized into two types: qualitative and quantitative (Fig. 1). Qualitative resistance (monogenic resistance) is when an abrupt change in a sensitive fungal population occurs that results in a distinct sub-population that is resistant to the fungicide at field use rates. The benzimidazoles typically show this type of resistance. Different levels of resistance (i.e., resistance factors) can still occur in individuals reflecting different mutations in the target β-tubulin gene. These changes result in substitutions of different amino acids and subsequent different binding potential of the fungicide to the β-tubulin molecule. Quantitative resistance (polygenic resistance) is when mutations of several genes each contribute to the development of resistance. Fungal populations respond to the fungicide selection pressure in a continuous shift from sensitive to resistant to highly resistant populations. This is because these mutations can be additive, resulting in an increased resistance factor. This results in decreased efficacy over time. The DMI fungicides typically show this type of resistance. Both types of resistance, qualitative and quantitative can occur in a single fungal species responding to fungicides with different modes of action. Monilinia fructicola and Podosphaera (Uncinula) necator show qualitative resistance to the benzimidazole and quantitative resistance to the DMI fungicides.
Fig. 1. Frequency distribution of EC50 values in fungal populations with no resistance, with qualitative resistance (e.g., MBC fungicides), or with quantitative resistance (e.g., DMI fungicides). Only one population with a distinct baseline range of sensitivities is observed in a sensitive population (no resistance). For qualitative resistance, a shift in fungicide sensitivity is observed by the presence of two distinct populations: a sensitive baseline population and a resistant population. For quantitative resistance, there is a gradual shift to increased EC50 values, resulting in a range of sensitivities within the population due to a step-wise accumulation of resistance genes. For both, qualitative and quantitative resistance, frequencies of resistant isolates as compared to sensitive isolates can vary widely (i.e., heights of each distribution may be different). Modified from Brent and Hollomon (2007)2.
Kendall and Holloman (1998)[i] stated that “Unlike insecticide resistance, with fungicides cross-resistance patterns generally follow modes-of-action, presumably reflecting target site alterations rather than uptake and detoxification changes.” Thus, the most effective way to combat fungicide resistance is to mix or alternate fungicides with different modes of action (classes of fungicides) and, if possible, at least one rotational mix partner should be a multi-site material. For this reason, the Fungicide Resistance Actin Committee (FRAC) has promoted a number system that is used to group fungicides within the same chemical class and with the same mode of action. This system simplifies resistance management practices to rotating fungicide usage between FRAC Code numbers.
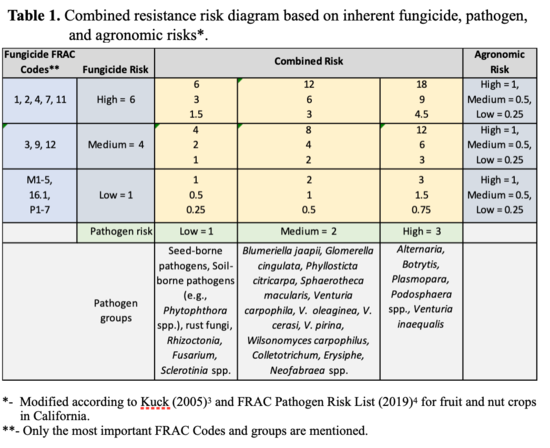
Factors determining the risk of fungicide resistance development in a pathogen population include: 1) fungicide chemistry; 2) fungal species; and 3) the agronomic practices (Table 1). Specific components of these factors can be outlined as follows for a pathogen causing disease on a susceptible host:
- 1. Fungicide Risk
- Single-site vs. multi-site mode of action compounds.
- Selection pressure: number of applications or the exposure frequency.
- Selection pressure: rate effect may be involved with certain types of fungicide resistance, such as quantitative resistance as opposed to qualitative resistance.
- Degradation of the fungicide over time under different environments
- 2. Pathogen Risk
- Inherent resistance frequency in the population (e.g., 10-4, 10-6, etc.)
- Comparative fitness of sensitive and resistant strains (survival attributes of the resistant population)
- Pathogenicity and virulence
- Propagation and survival
- Low efficacy, competition, and slow dispersal may help reduce but not prevent the development of resistance.
- 3. Agronomic Risk - an interaction of fungicide, environment, and usage practices: The stability of the fungicide on the plant and the interaction of the fungicide with the fungus under different environments and agricultural practices including host susceptibility (Fig. 1).
- Crop susceptibility
- Application volumes, equipment (air applications, airblast and electrostatic sprayers), frequencies, and methods (mixtures, alternate rows, etc.).
Conclusion: Resistance development is a complex process and has to be determined for each Pathogen-Fungicide-Agronomic practice.
The “recipe for resistance development” follows a general procedure in the lab: expose large numbers of propagules of the pathogen, expose the same population repeatedly to the same mode of action, and use low concentrations of the fungicides that may favor quantitative-types of resistance development. In the field, a parallel situation may occur:
1) Highly susceptible varieties under favorable environmental conditions generally support high populations of primary or secondary inoculum of the pathogen.
- Improper timing of fungicide application in respect to host stage, environmental conditions, or both.
- Application of fungicide after an epidemic occurs (high populations of the pathogen)
- Cultural practices that favor increases in pathogen populations (e.g., lack of pruning out cankers or infected tissue).
- Cultural practices that create environments that favor disease (e.g., long irrigation sets, irrigation designs that favor wetting of the canopy).
- Plant nutrition and fertilizer programs that favor development of susceptible tissue (e.g., high nitrogen fertilization programs).
2) Improper fungicide rate or application timing.
- Off-label rates are used or occur due to alternate row applications. This results in pathogen populations that are repeatedly exposed to low fungicide concentrations. This allows for survivors and resistance.
- Improperly timed applications due to environmental conditions. (e.g., alternate row 3- day re-application intervals delayed due to rain).
3) Repeated use of the same fungicide mode of action (Using one FRAC Code repeatedly in a growing season).
- Lack of awareness of FRAC codes, biological agents, or natural products available
- Poor understanding of IPM practices available.
- Other modes of action are not available on the commodity.
UC guidelines on fungicide resistance management can be described as following the “RULES” -
- Rotate between different fungicide modes of action as indicated by the FRAC number on each fungicide product (e.g., FRAC 7 should not be followed by FRAC 7; instead use FRAC 7, then follow with FRAC 3 or FRAC 3/11, FRAC 3/9, and FRAC 7/11).
- Use labeled rates – Fungicide labels often provide a range of rates: use the upper range for high disease pressure and the lower range for low disease pressure. Proper rates include proper coverage to minimize survivors from inadequate exposure to the toxicant.
- Limit the total use of any single-site mode of action fungicide to ideally one or two per growing season.
- Educate yourself about the mode of action, spectrum of activity, recommended rates, and the performance of a fungicide against various diseases. This information is found later in this document.
- Start a fungicide spray program with a multi-site mode of action fungicide, pre-mixture, or tank mixture to reduce the total fungal population that is exposed to any single-site mode of action fungicide used later in a sequence of fungicide applications. NOTE: Never use a single-site mode of action fungicide or a pre-mixture when high levels of disease already occur. The possibility of selecting fungicide resistant individuals is more likely to occur when high populations of a pathogen are being exposed to the selection pressure.
[1] Brent, K. J. and Hollomon, D. W. (1998) Fungicide Resistance: The Assessment of Risk FRAC Monograph No 2, Global Crop Protection Federation, Brussels, 48pp. http://www.frac.info/docs/default-source/publications/monographs/monograph-2.pdf
3 Kuck, K. H. (2005) Fungicide resistance management in a new regulatory environment. In: Modern fungicides and
anti-fungal compounds IV. Dehne, H. W., Gisi, U., Kuck, K. H., Russell, P. E. and Lyr, H. eds. BCPC, Alton UK., 35-43.
4 FRAC. 2019. Pathogen risk list. https://www.frac.info/docs/default-source/publications/pathogen-risk/frac-pathogen-list-2019.pdf.